Oxygen Control In Brewing

Intro:
Despite the best efforts of the brewing industry beer is, and remains, an inherently unstable product. Even beers produced at industry leading levels of in-pack dissolved oxygen, with reduced protein and tannin levels will still be stale towards the end of their often year long shelf life. Add, in less than ideal storage and transit conditions and staling can arise much faster than this.
As an industry we understand that beer is best consumed when it is as fresh as possible, this is the best way to ensure beer arrives at the final consumer as it was intended. Pressures from supermarkets which often require long life on products have been the key driver towards an ever increasing shelf life. This has contributed to the misguided view of some consumers that beer in small pack simply doesn’t go off.
There are signs that customer and consumer attitudes to beer shelf life are changing. Some larger companies are starting to embrace born on dates rather than best before dates to better indicate the age of the product to the consumer. The craft beer market has done much to emphasis this viewpoint and consumers familiar with the segment are now acutely aware of the issues that arise when trying to put long shelf lives on hoppy, hazy or unfiltered beers.
The chemical processes attributed to the changes seen in beer over its shelf life are varied and are by varying temperatures, exposure to UV light as well as levels of dissolved oxygen. The aim of the brewer is not to prevent these processes from occurring, but to slow or limit them to such a degree that the flavour profile of the beer remains acceptable for the duration of its shelf life.
What is beer staling?
Beer staling refers to the sensory and analytical changes a beer undergoes during storage. Whilst there are relatively few studies into the actual sensory changes observed during beer storage, work done by (Dalgliesh, 1977) presents a generalised view (see fig 1). These include a constant decrease in bitterness, an increase in sweet honey like tastes and aromas, increasing levels of an aroma of wet cardboard, and an increase and subsequent decline in a flavour described as ribes (the aroma of blackcurrant leaves). In addition to the taste and aroma changes, there is a subsequent decrease in colloidal stability as well as an increase in colour during storage.
It should be noted that most studies into the sensory and analytical changes that take place in beer during the aging process have looked at beers with a rather different sensory profile and colloidal stability to the hop-forward, and often hazy, beer produced by many craft brewers today. My own experience suggests that sensible additions to Dalgliesh’s chart would include a decline in dry hop aroma as well as a sometimes dramatic increase in colour in hazy, hoppy beers. The rapid rise of the craft brewing industry means that research into the staling of beers such as this has not kept pace with the overall growth of the industry. More work in this regard would be welcome.
Figure 1 Sensory changes arising during beer staling (Dalgliesh, 1977)
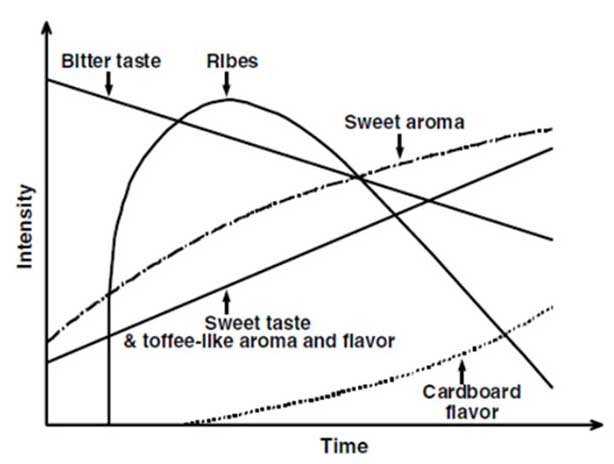 Figure 1 Sensory changes arising during beer staling (Dalgliesh, 1977)
Figure 1 Sensory changes arising during beer staling (Dalgliesh, 1977)
What causes beer staling
Ask the majority of brewers, what is the cause of beer staling, and their almost unanimous response would be oxidation. ‘Stale beer is stale because it has been oxidised’. The reality is rather more complicated! An in-depth review of the causes of beer staling is beyond the scope of this article, however I will try to outline some of the basic oxidative interactions below.
What is oxidation?
Oxidation is a way of describing a chemical reaction in which there is a loss of an electron from one atom to another. The atom which loses an electron is ‘Oxidised’. Conversely, the atom gaining an electron is ‘Reduced’, see Fig 2. Every time a molecule or atom is oxidised another atom or molecule must be reduced. This gives rise to the REDOX reaction. Those who studied Chemistry at school might remember the old mnemonic OIL-RIG, standing for oxidation is loss, (of electrons) and reduction is gain.
Oxidation can also be described in terms of the oxidation state of the atom. Electrons have a negative charge of -1 hence the loss of an electron increases the oxidation state by +1 for every electron lost. Reduction, where electrons are gained leads to a decrease in the oxidation state of the molecule by -1 for every electron gained.
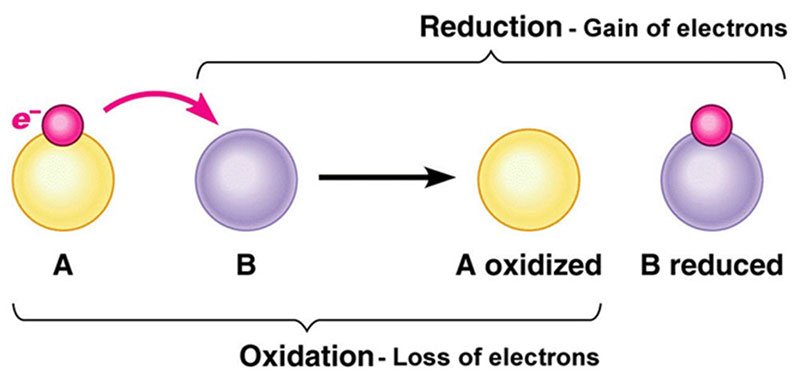
Figure 2- Oxidation
Generation of ROS
Many of the reactions recognised to cause staling can be attributed to the action of reactive oxygen species (ROS) in beer. Molecular oxygen is relatively unreactive in its ground state and so must first be activated to form the ROS responsible for these reactions. This activation is catalysed by the transition metals Iron and Copper and is an example of a Redox reaction. The amount of ROS generated is dependent on the level of oxygen in the beer, the storage temperature, as well as the availability of Iron and Copper ions to catalyse the reaction (Vanderhaegen, 2006).
Once generated ROS can go on to react with the many organic molecules present in beer including; alcohols, higher alcohols, hop bitter acids, polyphenols, amino acids and lipids. These oxidative changes result in many of the sensory changes seen during beer aging (Vanderhaegen, 2006).
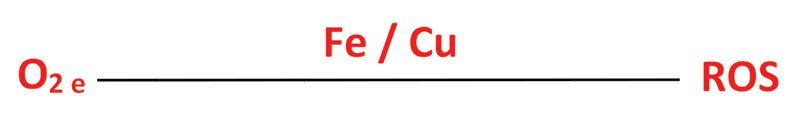
Figure 3- Creation of Reactive Oxygen Species (ROS)
E – 2 – Nonanal
After Oxidation the other most often quoted reason for an aged character in beer is the increase in a compound known as E-2-Nonenal or (Trans-2-nonenal). This compound is responsible for the wet cardboard aroma frequently detected in aged beers and is recognised as arising from the oxidation of malt derived lipids. Two mechanisms have been proposed for the creation of this compound, an enzymatic method involving the malt enzyme Lox-1, and a non-enzymatic method resulting from the reaction of lipids with ROS (Vanderhaegen, 2006). Importantly, this interaction is thought to take place during hot side brewing processes and the increase in cardboard flavour in final pack is due to the subsequent release of bound forms of the chemical.

Figure 4-Creation of E-2-Nonenal
Methods for control of oxidised character
Strategies for reducing the tendency of a beer to stale quickly are based around the reduction of oxidation reactions by limiting the availability of molecular oxygen and transition metal ions as well as slowing the subsequent generation of ROS. Methods to reduce E-2-Nonenal pick up focus on reduced Lox-1 enzyme activity as well as reducing oxygen ingress to hot side processes.
Methods to reduce oxidation reactions in finished beer
Limiting dissolved oxygen concentration in pack is crucial to preventing the uptake of stale character in beer. To this end there are various strategies the brewer can employ to reduce the final level of dissolved oxygen in pack. Meticulous control of oxygen uptake after fermentation is key. All pipes and filters should be flushed with CO2 or DAL prior to making beer transfers and, if possible, the oxygen content measured. Seals and fittings should be tight and well maintained and bright beer tanks should be cleaned under an inert atmosphere. Metal ions should be kept to a minimum by using all stainless steel pipework and fittings and quality crown caps. Technologies such as oxygen scrubbing crown caps should also be considered.
In addition to these physical strategies thought should be given to additions that could be made to the product to protect it from staling during its shelf life. The two key strategies here are the addition of bottle conditioning yeasts as well as antioxidants.
Bottle conditioning yeasts such as the Lallemand CBC-1 strain can protect beer through a variety of mechanisms. First, viable yeast has a strong ability to metabolise molecular oxygen reducing its level in pack and preventing the generation of ROS. In addition, yeast is known to create the antioxidant sulphite which can react with, and prevent, the onward reaction of ROS as well as binding with flavour active staling aldehydes to create flavourless products (Guido, 2016). Finally, active yeast and a strong secondary fermentation have been shown to reduce existing staling aromas by reduction of flavour active staling compounds in beer (Saison, 2010).
Antioxidants blends such as Lallemand Vicant SB, which contain sulphites as well as other antioxidants, can also help prevent beer staling. The mechanism is much the same as the antioxidant effect provided by bottle conditioning, binding flavour active staling compounds, but also preventing the reaction of ROS with polyphenols, sugars and amino acids which tend to cause colour pick up and hazes, see Fig 5. An addition of 2-4g/hl to final beer is sufficient to improve flavour and colloidal stability as well as reduce browning.

Figure 5 – Using Vicant SB to reduce beer staling
Methods to reduce E-2-Nonenal pickup
Limiting the creation of E-2-Nonenal focusses on the hot side of the brewing process. The two accepted routes to its creation involve an enzymatic oxidation route and an auto oxidation route due to the action of ROS in the mash and wort. Approaches to the prevention of the enzymatic route involve limiting the availability of the Lox-1 enzyme by the use of barley varieties with low levels of the Lox-1 enzyme, as well as higher temperature mashing and kilning regimes (Vanderhaegen, 2006). Mashing at lower pH and milling regimes which prevent embryo damage have also been explored (Vanderhaegen, 2006). Limiting the availability of molecular oxygen needed for the Lox-1 reaction by anaerobic mashing and milling processes, as well as purging grist and mashing vessels has also been shown to reduce E-2-Nonenal production (Vanderhaegen, 2006).
Preventing the auto oxidation route could involve all of the above techniques for limiting oxygen uptake into the mash and wort but also the use of antioxidants such as Lallemands Vicant SBX to help prevent the reaction of lipids with ROS. An addition of 5-10g/hl to the mash is sufficient to improve flavour and colloidal stability as well as reduce browning.
To sum up
Beer staling is a complex process involving many different pathways. One of the key reaction paths is the reaction of reactive oxygen species (ROS) with the numerous organic molecules present in beer. Strategies for prevention of these reactions focus on the reduction of dissolved oxygen concentration in final beer but could also include the use of specialist bottle conditioning yeasts, as well as antioxidant additions to the beer and to upstream hot side processes.
Bibliography
Dalgliesh, C. (1977). Flavour Stability. European Brewery Convention Congress, (pp. 623-659).
Guido, L. F. (2016). Sulfites in beer: reviewing regulation, analysis and role. Scientia Agricola, 73.
Meilgaard, M. (1972). Stale flavour carbonyls in brewing. Brewers Digest, 47, pp. 48-57.
Saison, D. (2010). Decrease of Aged Beer Aroma by the Reducing Activity of Brewing EYast. Journal of Agricultural and Food Chemistry, 58, 3107-3115.
Vanderhaegen, B. (2006). The Chemistry of Beer Aging – A Critical Review. Food Chemistry, 95, 357-381.



